What Term Is Used to Describe Uranium Uranium-238
It provides nuclear fuel for the generation of electricity. What term is used to describe splitting a large atomic nucleus into two smaller ones.

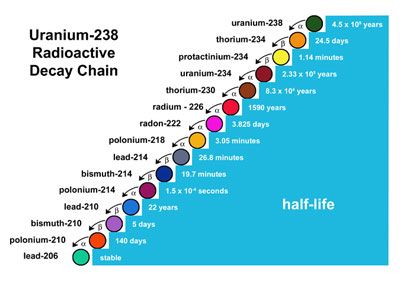
Scheme Of Uranium U 238 Decay Series Download Scientific Diagram
3Potassium iodide is a frequent salt additive and it is often given to populations.

. Natural uranium contains 07. Uranium 238 which alone constitutes 9928 of natural uranium is the most common isotope of uranium in the nature. The key difference between Uranium 234 235 and 238 is that Uranium 234 contains 142 neutrons and Uranium 235 contains 143 neutrons whereas Uranium 238 contains 146 neutrons.
Because this method is useful for the period of time from about 100000 years to 1200000 years before the present it helps in bridging the gap. Uranium 238 wikipedia alpha decay particles consist of two protons atomic nuclear chemistry monday 102912 prep 1 find ch103 chapter 3 radioactivity and equations 10 when henri becquerel placed beta equation tessshlo radioactive processes ppt stickman physics Uranium 238 Wikipedia Alpha Decay Particles Consist Of Two Protons Atomic Nuclear. Uranium-238 and Uranium-235 are the parent isotopes of decay chains that can be used to provide a chronology back to 500 ky.
The process used to. 7038108 years Uranium 238. The weight percentage for U-235 is 072 and 0.
Uranium is generally used in reactors in the form of uranium dioxide UO 2 or uranium metal. An electrically neutral uranium-235 isotope contains 92 electrons 92 protons and 143 neutrons ie. The decay chain of 238 U is commonly called the radium series sometimes uranium series.
Uranium-235 is considered infertile and Uranium-238 as fertile because it can be converted to Plutonium another radioactive substance. It is an abundant source of energy that has been used for about 60 years. Natural uranium contains 3 radioactive isotopes U-234 U-235U-238.
Uranium 238 has the longest half-life 44710 9 years and therefore its abundance is so high. Uranium-235 can initiate a fission chain reaction but Uranium-238 cannot. Astatine bismuth lead polonium protactinium radium radon thallium and thorium.
Uranium is a heavy metal. So its atomic number is 92 and mass number is 235. Uranium 235 and uranium-238 accounting for 99 of the uranium on the planet are the most common naturally occurring uranium isotopes.
Further most civilian and many military reactors require uranium that has a higher proportion of uranium-235 than present in natural uranium. The processing of uranium for industrial and governmental use changes the ratios of the different isotopes. The radioactive associated with.
Uranium-234uranium-238 dating method of age determination that makes use of the radioactive decay of uranium-238 to uranium-234. If the fraction of 235 U is increased it is called enriched uranium. Answer 1 of 4.
This isotope has the longest half-life 44710 9 years and therefore its abundance is so high. B An atom of plutonium-239 contains 145 neutrons. E 92 p 92 n 146.
What term is used to describe splitting a large atomic nucleus into two smaller ones. The leftover 238 U also known as depleted uranium DU is approximately 40 less radioactive than natural uranium ore and can be used in munitions. Uranium 238 which alone constitutes 9928 of natural uranium is the most common isotope of uranium in nature.
Uranium enrichment separates these so the 235 U can be used as nuclear fuel. A Uranium-238 is the most common isotope of uranium and can be represented using this symbol. Which of the following statements regarding uranium-238 and plutonium-239 is false 120.
Uranium 238 is a fissionable isotope but is not a fissile isotope. The half-life of uranium-235 is 713000000 years the half-life of uranium-238 is 4500000000 years b. C An atom of plutonium-239 contains two.
Beginning with naturally occurring uranium-238 this series includes the following elements. The half-life of uranium-235 is 713 seconds the half-life of uranium-238 is 45 minutes c. Uranium 238 is transformed in thorium 234.
Briefly describe the process of decay for uranium-235 and uranium-238. This process of increasing the amount of U-235 in the natural Uranium is known as Uranium enrichment. 1Why are other radioactive isotopes like uranium-238 uranium-235 iodine-131 dangerous to human health.
Uranium U ore as mined is approximately 99 238 U and 1 235 U Rella 2015. 120 In a certain type of nuclear reactor uranium-238 can be converted to plutonium-239 another radioactive element with an atomic number of 94 that can be used to power nuclear reactions. The photograph shows a glass plate made from uranium glass.
What are the half lives for uranium-235 and uranium-238. 2Nuclear power plants use uranium-235 instead of the more abundant uranium-238. E 92 p 92 n 143.
The method can be used for dating of sediments from either a marine or a playa lake environment. The half-life of uranium-235 is 713 days the half-life of uranium-238 is 450 days d. However if the portion of 235 U is decreased it is called depleted uranium.
In an old system 500 ky a radioactive secular equilibrium is established between the parent 238 U or 235 U and their daughter radioisotopes. U-238 cannot be converted directly to U-235 but the concentration of U-235 in the natural Uranium can be increased. An isotope of uranium of mass number 238 that is the most stable uranium isotope that constitutes over 99 percent of natural uranium that is not fissile but can be used to produce a fissile isotope of.
It is a weakly radioactive element whose half-live ranges from 159200 years to 45 billion years. 238 U belongs to primordial nuclides because its half-life is. Definition of uranium 238.
Natural processes can disrupt this equilibrium and the subsequent in. Nuclear weapons use the metallic form. 238 92 U 1 State what information the numbers 92 and 238 give about the nucleus of this isotope of uranium.
Production of uranium dioxide or metal requires chemical processing of yellowcake. Uranium is a silvery-grey metal that is radioactive. In years what is the half life of uranium 235 and 238.
An electrically neutral uranium-238 isotope contains 92 electrons 92 protons and 146 neutrons ie. It is the ninety-second element on the periodic table. Depleted uranium is less radioactive than natural uranium and enriched uranium is more radioactive than natural uranium.
O Laura HealeyShutterstock Uranium oxide is used to give the glass a green colour. 238 U belongs to primordial nuclides because its half-life is comparable to the age of the Earth 4510 9 years.

The U 238 Decay Chain Download Scientific Diagram

Uranium 238 Radioactive Chain Diagram The Radioactive Chain Begins Download Scientific Diagram

Enrichment U 238 More Than 99 Of The Uranium In The Ground Is U 238 U 238 Doesn T Fission Ppt Download
Comments
Post a Comment